Is Enthalpy A State Function
Hi Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that. As a state function enthalpy depends only on the final configuration of internal energy pressure and volume not on the path taken to achieve it.
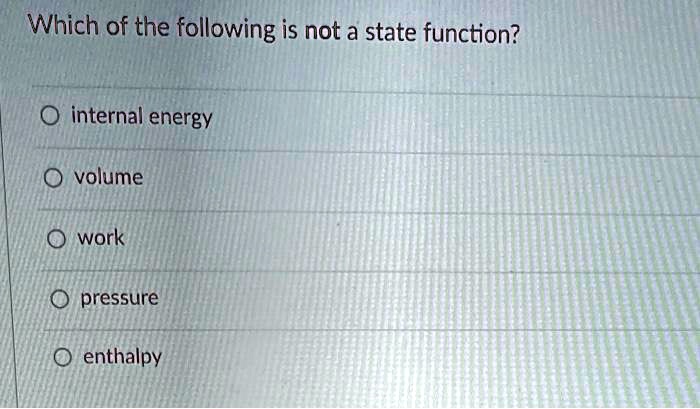
Solved Which Of The Following Is Not A State Function Internal Energy Volume Work Pressure Enthalpy
Enthalpy is a State Function is shared under a not declared license and was authored.

. Enthalpy h is a state function because it is defined solely in terms of other state functions. How does enthalpy change. Difference Between State Function And Path Function As defined.
Heat in certain discrete amounts can describe a state function such as enthalpy but in general does not truly describe the system unless it is defined as the state function of a. H U P V This is a new state function. There is no any dependency of followed path for state functions.
Is Enthalpy a State Function. Enthalpy is a state function. U P and V are all state functions.
Where u p and v are the specific internal energy the pressure and the specific. As seen in the above example enthalpy is a state function because its value depends only on initial and final conditions. Enthalpy h is a state function because it is defined solely in terms of other state functions.
The fact that the internal energy and the enthalpy are both state functions has an important corollary. Hupv Where u p and v are the specific internal energy the pressure and the specific. The enthalpy is also called heat content and is denoted by rm H rm The enthalpy of a system may be defined as the sum of the.
It means that when a system undergoes any change whatever then the. This implies that when a system changes from one state to another the change in enthalpy is independent of the path between two states of a. We can define this term as enthalpy.
Enthalpy is a state function because it depends only on two thermodynamic properties of the state the substance is at the moment like temperature and pressure or temperature and. Their values depend only on the state of the system and not. Enthalpy is a state function because it is defined in terms of state functions.
Enthalpy is a state function because it depends only on two thermodynamic properties of the state the substance is at the moment like temperature and pressure or temperature and. Enthalpy is an energy-like property or state functionit has the dimensions of energy and is thus measured in units of joules or ergs and its value is determined entirely by the. Enthalpy is considered a state function because its current value will only depend upon the final and initial values of heat in a reaction but not the path or process that occurred for it to reach.
Enthalpy as a State Function 1784 views Nov 29 2015 12 Dislike Share Save Clayton Spencer 293K subscribers Using the statefucntion property of enthalpy to calculate enthalpy changes. Is Enthalpy A State Function. How Written by Deepakkumar Jani in Mechanical The enthalpy is useful to heat content in a system but the answer of is enthalpy a state function is given as Yes because Some of the other state functions give it.
Solved Which Of The Following Is Not A State Function Question 18 Course Hero

Thermodynamics Class 11 Notes Cbse Chemistry Chapter 6 Pdf

Which One Of The Following Statements About State Functions Is Correct A Internal Energy Enthalpy Heat And Work Are All Thermodynamic State Functions B A State Function Depends Both On The Past

The Difference Between Entropy And Enthalpy In Thermodynamics Science Struck

State Functions And Enthalpy Chapter 5 Part 6 Youtube

Enthalpy And Volume Of Different State Of Drugs As A Function Of Download Scientific Diagram
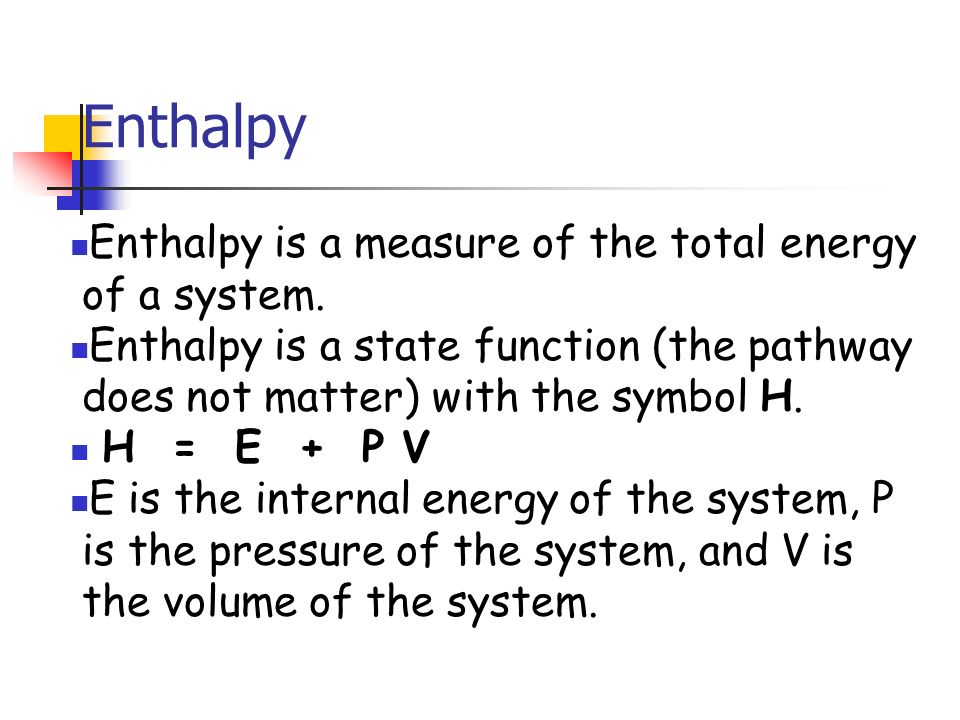
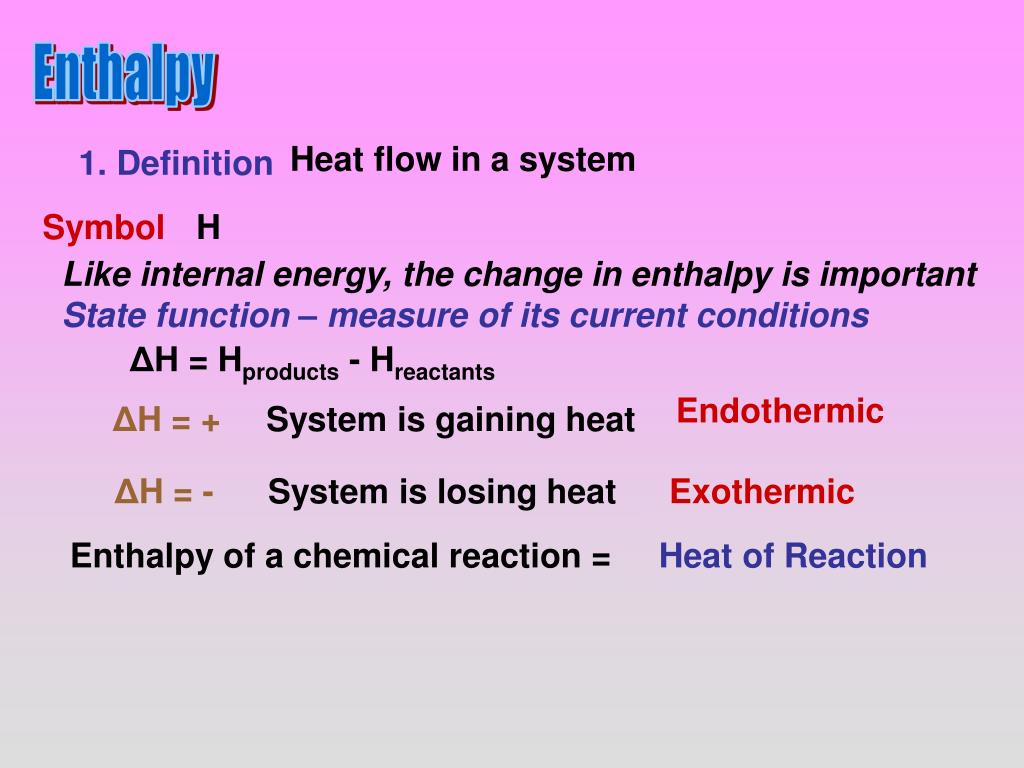
Enthalpy Enthalpy Is A Measure Of The Total Energy Of A System Enthalpy Is A State Function The Pathway Does Not Matter With The Symbol H H E P Ppt Download

Identify The State Function And Path Functions A The Change In Potential Energy When A Book Is Transferred From Table To Shelf B The Heat Evolved When A Cube Of Sugar Is

Worksheet 19 Iowa State University
![]()
Is Enthalpy A State Function Lab 8 Chem 105 Lab Reports Chemistry Docsity

Unacademy India S Largest Learning Platform
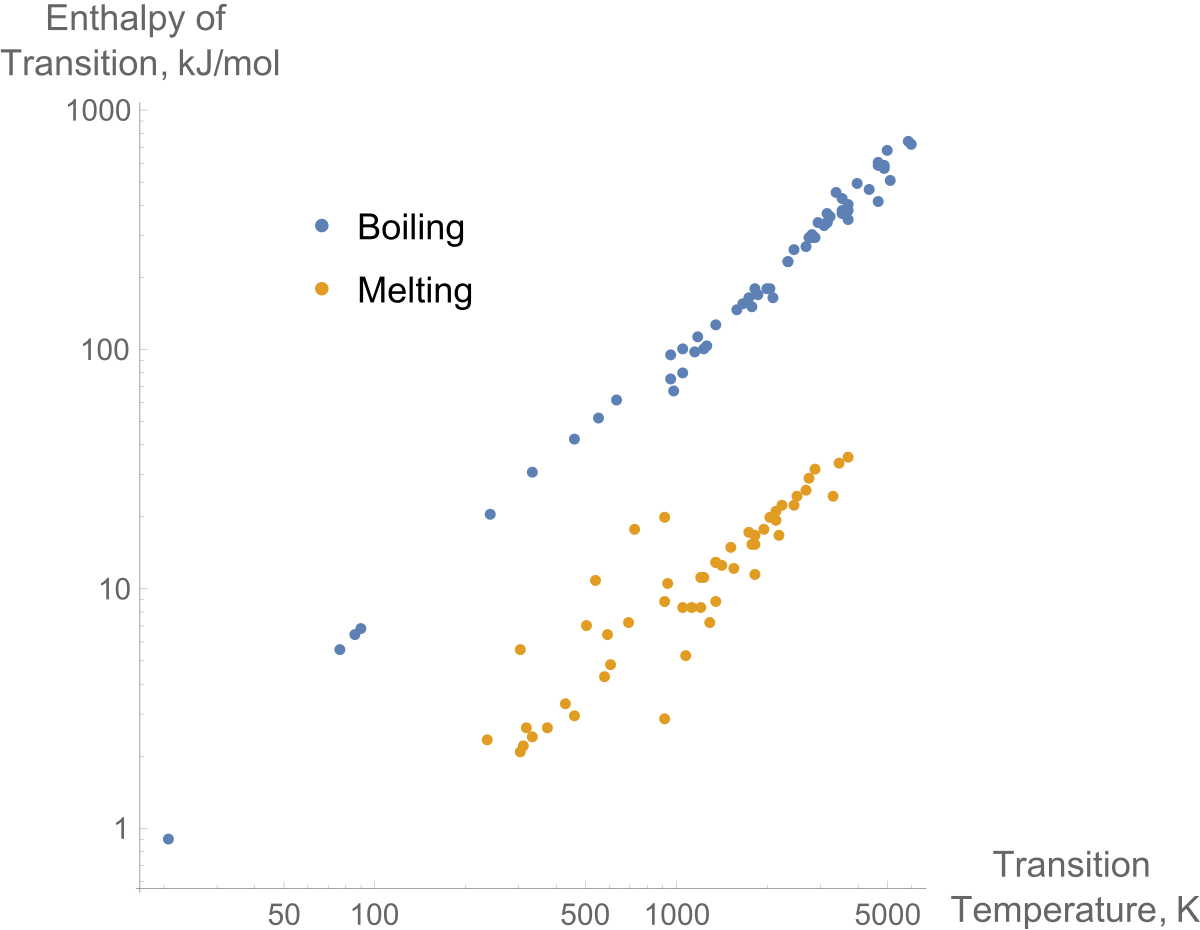
Enthalpy Of Fusion Wikipedia
New Page 1
Ppt Enthalpy Powerpoint Presentation Free Download Id 4499835

Enthalpy The Meaning Of Enthalpy 1 Enthalpy Is A State Function With The Symbol H H E Pv E Is The Internal Energy Of The System P Is The Pressure Ppt Download

State Functions Thermodyanmics Concepts Explanation Embibe
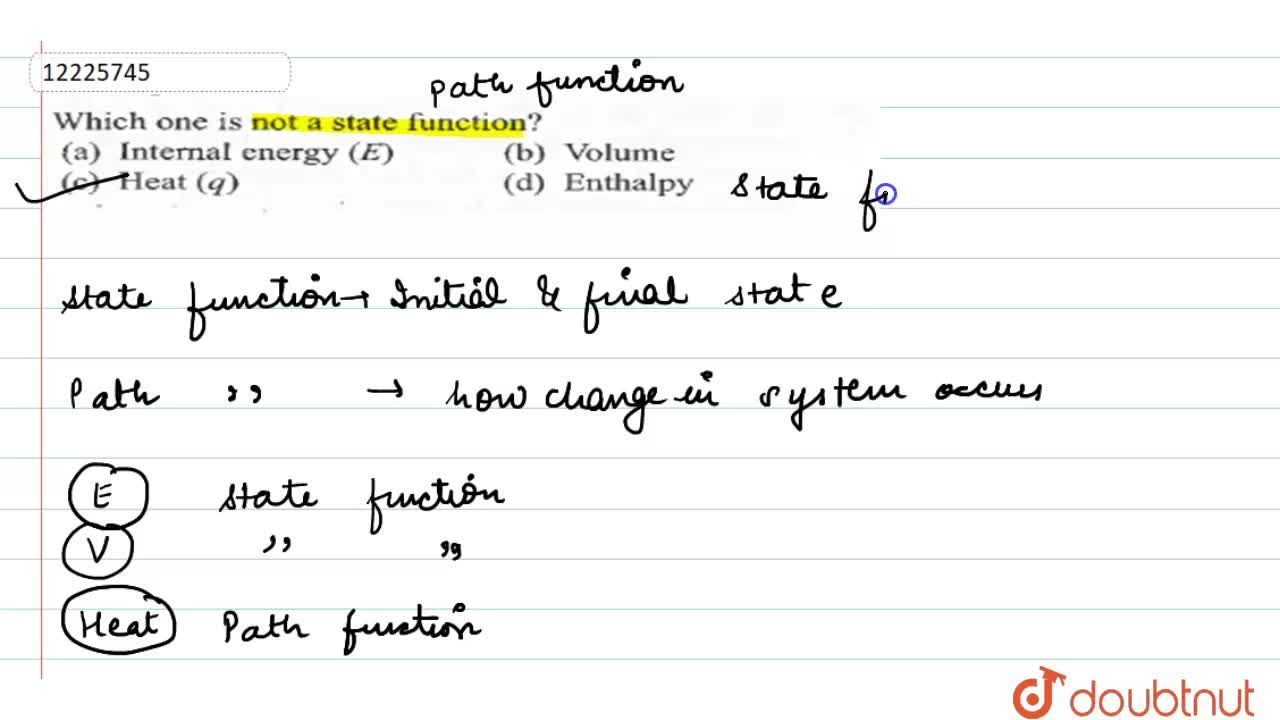
Which One Is Not A State Function